How to Determine Solubility in Water of Organic Compounds
Propranolol oxprenolol metoprolol and timolol are the most lipid-soluble beta-adrenoceptor antagonists and atenolol nadolol and sotalol are the most water-soluble. A common rule with solubility is like.

Chemistry The Central Science Chapter 13 Section 3
Unless the solid is already a fine powder crush a small amount of the solid on a watch glass with the back of a spatula.
. Previously you were introduced to guidelines for predicting the solubility of ionic compounds in water. Place about 1mL of the. Do not weigh the solid.
For complete oxidation of organic matter it will take about 20. There are more than 100 compounds in water that have been listed in the literature as toxic organic compounds 11 22. The higher the lattice energy the more polar the solvent must be to overcome the lattice energy and dissolve the substance.
Simply use enough to cover the tip of a small. Recrystallization is the primary method for purifying solid organic compounds. The impurities may include some combination of insoluble soluble and colored impurities.
Limitation of BOD measurement. Urea is the chief nitrogenous end product of the. This is an important issue in biology because water is the solvent in living systems.
Water molecules H 2 O have an unusual structure which makes them similar to a magnet. Urea has important uses as a fertilizer and feed supplement as well as a starting material for the manufacture of plastics and drugs. Procedure for Determining Solubility of Organic Compounds The amounts of material to use for a solubility test are somewhat flexible.
These compounds include insecticides pesticides solvents detergents and disinfectants 11 21 22. Further information about each of the solubility tests follows the procedure. Most organic compounds have covalent bonds while most inorganic compounds have ionic bonds.
Some ionic compounds arent stuck together very. A solution of 50 grams of santonic acid in 300 grams of benzene boils at 8191C. BOD DO1-DO5 dilution factor temperature factor.
Water H 2 O is nonflammable nontoxic cheap and will dissolve many polar organic compounds. Solubility is a physical property of organic compounds because the chemical composition or nature of the compound does not change when dissolved in solution. To obtain a pure compound these impurities must be removed.
Water 15M HCl concentrated H 2 SO 4 06M NaHCO 3 25M NaOH and organic solvents. In fact it is miscible. As an example perform such a calculation to find the molecular mass of the organic compound santonic acid which dissolves in benzene or chloroform.
A flowchart showing the sequence of solubility tests along with the appropriate conclusions is shown in Figure 1. Light must be excluded to prevent the growth of algae that may produce O2 inside bottle. They will not be found naturally in water.
Lipid solubility 13 determines the extent to which a drug partitions between an organic solvent and water. To determine BOD water sample is placed in a 300ml BOD bottle seal it and incubated at 20C for 5 days in dark room. They are usually man-made pollutants.
Urea also called carbamide the diamide of carbonic acid. The solubility of organic compounds nearly always increases with temperature. Each is removed in a separate step in the.
Its formula is H2NCONH2. Also present are a large number of compounds that either have not been identified or their effects on health have not been characterized. Because of its high polarity water is the most common solvent for ionic.
Raw water sources and disinfected water supplies may contain organic compounds that have been demonstrated to be carcinogenic or otherwise toxic in experimental animals or in epidemiological studies. Compounds obtained from natural sources or from reaction mixtures almost always contain impurities. For condensed phases solids and liquids the pressure dependence of solubility is typically weak and usually.
The ionic bonds allow inorganic compounds to dissociate into positive and negative ions in. Palatability viscosity solubility odors and chemical reactions are. Consider the water solubility of the following compounds of comparable size and molecular mass.
When you drop an ionic compound in water these water magnets will gather around it trying to pull the positive and negative ions apart. Its drawback is high boiling point 100 C 212 F making it relatively nonvolatile and difficult to remove from crystals unless they are vacuum-dried in a desiccator. The technique of recrystallization used for purification of solids depends on a solutes different solubilities in hot and cold solventA few exceptions exist such as certain cyclodextrins.
One end has a positive charge while the other has a negative. Of these compounds the alcohol 1-propanol is most soluble. One valuable use of these relationships is to determine the molecular mass of various dissolved substances.
Acebutolol and pindolol are intermediate 14. To illustrate lets consider the water solubility of organic compounds. Ionic substances are generally most soluble in polar solvents.
It is a colourless crystalline substance that melts at 1327 C 271 F and decomposes before boiling. The common solvents used to determine solubility types are. Use 2-3 drops of a liquid or approximately 10 mg of a solid.

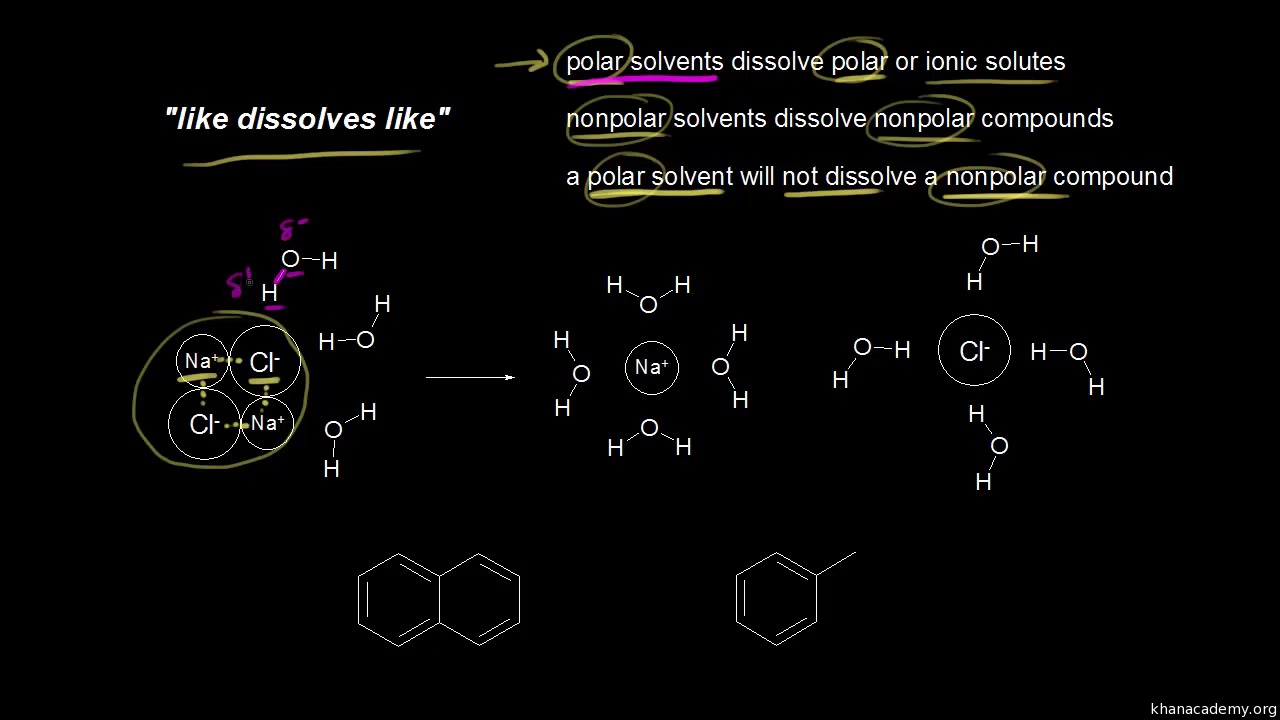
Solubility Of Organic Compounds Video Khan Academy

Solubility Of Organic Compounds Video Khan Academy

How To Predict The Water Solubility Of Organic Molecules Youtube
No comments for "How to Determine Solubility in Water of Organic Compounds"
Post a Comment